Anarchy in administering plasma therapy
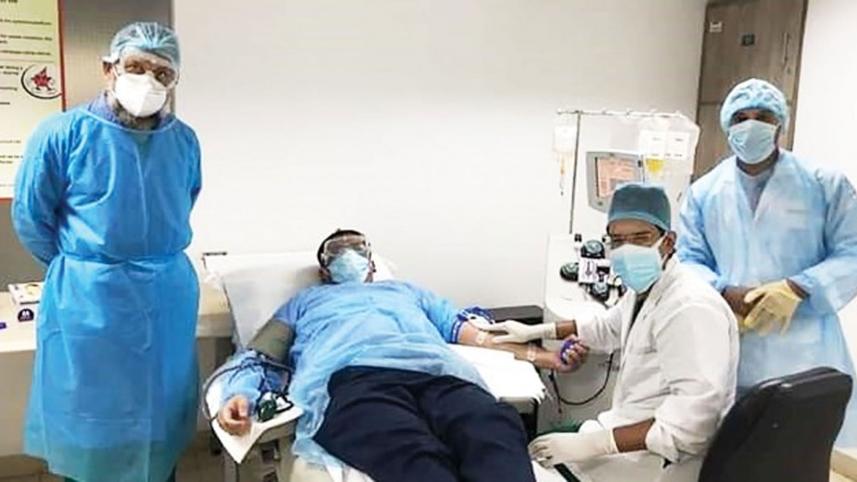
Covid-19 has compelled countries to test, try and come up with new treatments to cure the afflicted. We have been clutching at every straw, because the virus has defied and resisted many known medications. Clinical trials have only been approved in a few countries, but that is being done under well spelt-out and clear guidelines. In Bangladesh plasma therapy has been approved recently, but, reportedly, it was being used by many hospitals well before the treatment was approved by the authorities, with, regrettably, calamitous results in many cases.
Approval has been given last month, to three hospitals, namely, Dhaka Medical College Hospital (DMCH), Bangabandhu Sheikh Mujib Medical University (BSMMU) and Rajarbagh Police Hospital—to conduct randomised clinical trials of plasma therapy for Covid-19 patients, but doctors are still not sure as to when exactly to administer the therapy. What we see now, and which is the lament of all the experts, is complete chaos in respect of this particular mode of treatment. There are so many factors that need to be considered and so many if and buts, which the hospitals must be made aware of. It is rather alarming to hear experts describe the current manner of applying the therapy as unethical. How can one determine the fitness of the donor without carrying out antibody test, for example?
We fail to understand why, if the relevant persons in the health ministry and its agencies are doing their jobs honestly to justify their keeps, should it take a month to come out with a guideline to a treatment procedure which should have been accompanied with clear instructions to start with. One wonders what to make of the statement of the head of the national technical advisory committee on coronavirus that decision to whether a guideline will be formed will be taken only after the results of randomised clinical trials are in hand. The trial needs a procedure guideline too, one would have thought. A suggested proposal from the DMCH was sent to the national technical advisory committee over formulating a policy on plasma therapy on June 20, without any response as yet.
Actions intended for public good can be counterproductive unless it is well thought out. In this case it can cost lives. Will the technical committee please wake up?



 For all latest news, follow The Daily Star's Google News channel.
For all latest news, follow The Daily Star's Google News channel.
Comments